398 Greenlight Guru Quality Management System Reviews
Overall Review Sentiment for Greenlight Guru Quality Management System
Log in to view review sentiment.
Greenlight Guru is an easy-to-learn eQMS with an intuitive interface complemented with an exceptional question database with instructions for any skill level. If any question is left unanswered, you are assigned a customer success representative during onboarding who is available with quick responses. Trainsitioning to the program and training employees was an extreemly easy process. Review collected by and hosted on G2.com.
The document uploading process takes time due to not having a bulk upload feature, so if transitioning to the software from an established paper QMS, be aware of the time it may take to add everything to the database. They do offer services to assist. Review collected by and hosted on G2.com.

• User-friendly interface, robust and reliable document management capabilities that definitely make it not only a valuable tool for ensuring product quality but also to comply with regulatory requirements.
• The ability of keeping up with end-user needs, and suggest software updates & new features and enhancements, this helps to constantly improve efficiency of our company quality management system. Review collected by and hosted on G2.com.
• Greenlight Guru can be expensive, the subscription-based pricing model can be costly over time compared to one-time purchase software, which might be a barrier for small or startup companies with limited budgets.
• The predefined templates may not be flexible enough for companies with unique processes.
• Functionality Gaps: certain advanced features (e.g. training requirements) are missing integration of the old training approach (e.g. training events), making it more difficult to perform the transition. Review collected by and hosted on G2.com.
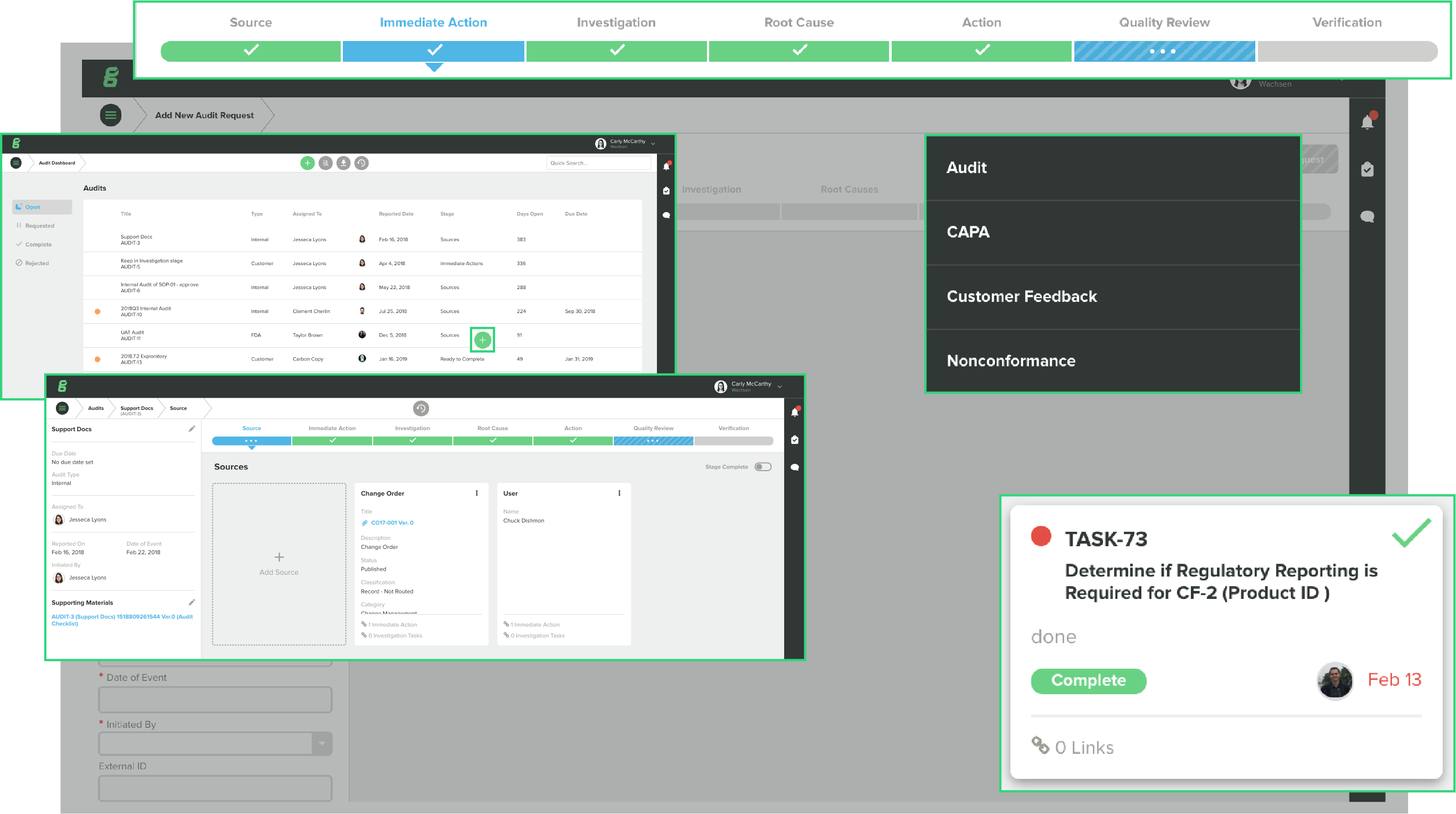
Automatic version control of documents. Task list for "to do's". Publish date for documents. Control multiple document changes using one change control number.
Adding: nonconformance and CAPA tracking - tasks tied to document changes. Also project and risk management is good, if you take the time to think through and set up everything carefully. Review collected by and hosted on G2.com.
It's a little confusing to start, moving from an existing paper-based, fully formed system to implementing it in GG. No in-app editing of documents; must download and re-upload. Review collected by and hosted on G2.com.
-“One-stop” qms tool that can replace a variety of disparate tools and processes. Incorporation of the “dashboard” allows user to review variety of open actions in a single place.
-For our largest product development project, the Risk Management Module was particularly useful. While some aspects took a little getting used to, the single tool greatly simplified the multitudes of related spreadsheets typically required.
-Straightforward document control. Review collected by and hosted on G2.com.
-Change orders must contain documents and cannot manage items alone without a document also involved
-Many early issues with the Products module did require significant interactions with the GG support team, but in the end most of these issues were resolved.
-BOM output customization is lacking. It would be beneficial to be able to include custom fields/attributes within the BOM output.
-The search function seems to work only intermittently, with issues distinguishing part numbers and other custom titles.
-Item attachments are not locked upon release of the item. After release, attachments can be deleted or changed. Review collected by and hosted on G2.com.

The Main advantage to use Green light Guru (gg) is to have all the SOP's, QA , Forms in one place and be able to update, review and have training management set up in one place.
It makes the training more efficient, helps keep track of the CAPA's and monitor the progress in a better way. Review collected by and hosted on G2.com.
It is cumbersome in the initial stages or during migration from one system to gg etc.
The email updates can not be sorted, you get a barrage of emails with updates from all the departments, though not related to you or even when you are not involved. Review collected by and hosted on G2.com.
As a quality supplier/customer; I love the automations and ease of adding automations. Love that documents are easily accessible. Review collected by and hosted on G2.com.
No real downsides that come to mind. I would like to see a Supplier section for audits, evaluations, report cards, etc. Review collected by and hosted on G2.com.
The favorites section, or "starred", is very helpful to keep track of the SOPs that I access the most frequently! I also like that certain keywords are searchable, even if they're not included in the title. Review collected by and hosted on G2.com.
I wish that some more keywords were searchable. I often forget the entire SOP code. Review collected by and hosted on G2.com.
1. Simple and effective doc control system
2. The new risk management tool is effective and easy to use
3. Prompt support from Gurus when needed - note: we did pay extra for a customer support package Review collected by and hosted on G2.com.
1. The Product Workspace is not integrated with the Doc Control Workspace
2. The Product Workspace approval process is separate from the Change Order approval process
3. The Product Workspace does not "lock" attachments after an item is approved by the team, this makes it difficult to appropriately manage revision control for design files associated with a device's components and subassemblies. Review collected by and hosted on G2.com.
Upsides include:
- GG Customer Service, Account Management, and Product Designers are consistent, solicit VOC, and are responsive
- all required QMS procedures in a single software platform
- relatively easy to implement
- user acceptance
- continued support from GG Review collected by and hosted on G2.com.
Items to (still) work on:
- please, please, please come up with a training matrix
- add ways to help prove training competencies (e.g. quiz's)
- help make it easer to get quality events into the system
- adjust the viewing permissions of lite users and consider adding temporary or limited time users (e.g. interns) Review collected by and hosted on G2.com.
1. Common Workflow for various QMS elements Review collected by and hosted on G2.com.
1. Training Workflow: Add "competency based" testing question to provide pass/fail score.
2. Workflow needs to talk to each other. Example: If NC workflow is required CAPA than NC workflow allow to open CAPA from NC workflow vs going to CAPA workflow to start. When NC open from CAPA than CAPA workflow "source" should state NC.
3. It is good to have "pre" and "post" quality approval in each work flow. Example: Audit work flow. Audit is completed is required "pre approval"= Execution of Audit. Once all audit findings are closed than final "post- approval" of Quality review.
4. Supplier module to include: Supplier file mainteance ( ISO, Audit, Quality Agreement) and Incoming Inspection module to allow upload actual incoming record for each material.
5. Change Control: Add 5th question for evaluation: Capture additional information as needed due to not every change all 4 questions address the business and compliance need. Review collected by and hosted on G2.com.