Clinical investigators used to rely on traditional paper case report forms (CRFs) to collect data from clinical research study participants.
Once the study was completed, the paper forms were transported to a different location for review, where the data was manually transcribed, cleaned, and prepared for analysis. However, this process was time-consuming and error-prone.
Many companies are now adopting electronic data capture (EDC) systems that offer a more efficient and accurate way to collect, manage, and study data to address these challenges. These systems allow users to design electronic case report forms (eCRFs) and a user-friendly interface for participants to work on.
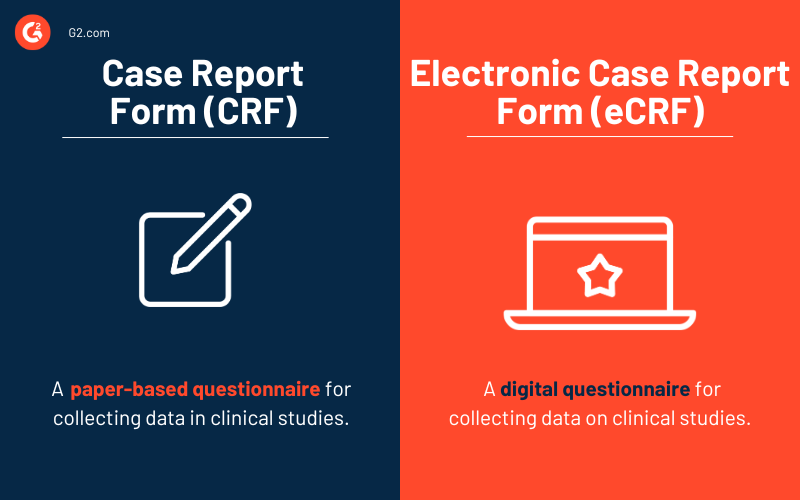
What is an electronic case report form (eCRF)?
An eCRF is a digital questionnaire created by clinical trial sponsors to collect data about a clinical study from research participants. The data collected from eCRFs is then analyzed to identify patterns and draw conclusions.
With eCRFs, participants enter directly into a computer database, eliminating the need for manual transcription and reducing the risk of errors.
This article covers all the basics of eCRFs. You'll learn what they are, how to design one, and the benefits of choosing eCRFs instead of paper-based CRFs for your subsequent studies.
CRFs vs. eCRFS
Clinical trials involve collecting a large amount of data, and how sponsors capture it significantly impacts the study's success. There are two primary methods for data capture: paper CRFs and eCRFs.
The advantages of eCRFs continue to grow, quickly overtaking paper CRFs, even though there are some potential obstacles to eCRFs, such as the need for more availability of on-site technology and comparably higher costs.
Paper CRFs are well suited for small studies or studies with varying designs, whereas eCRFs are considered if studies are extensive with similar designs. While paper CRFs don’t often require user training, clinicians need essential training and experience before implementing eCRFs are used in research.
Electronic data capture software is designed for collecting data in CRF clinical trials. It provides an efficient approach to data collection and management, whereas an electronic case report form is a digital questionnaire within the EDC software that facilitates the collection of specific data points. It allows CRF researchers to quickly and accurately gather information for analysis and reporting.
Designing a paper CRF is a tedious job that often requires manual data transfer from the source document to the paper, resulting in data errors. On the other hand, an eRCF provides data quality and reduces time spent on data cleaning. The system automatically generates redundant data such as protocol ID, site code, subject ID, and patient initials, ensuring no duplication of CRF pages.
Want to learn more about EHR Software? Explore EHR products.
Components of an electronic case report form
Here's a list of the various components and terminology used when creating eCRFs.
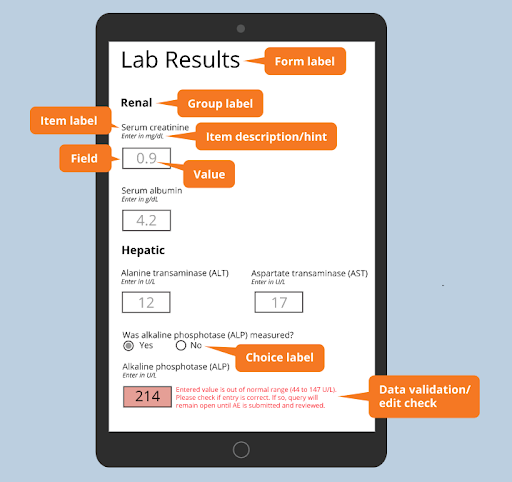
Source: OpenClinica
- Choice label: The unique labels in a multiple-choice question.
- Data validation/edit check: Inspects if the entered data meets specific criteria set by the form designer.
- Form label: A clear and concise title describing the questions in the form. This helps quickly identify and differentiate between forms.
- Field: The blank box where users input the required information.
- Group label: A sub-title used to categorize related questions in a form. It's beneficial for forms that contain a large number of questions.
- Item label: A short prompt that tells the user what information to enter into a specific field.
- Item hint: The instructions that provide more details about the item label or the information to be entered.
- Value: The data entered into the field. It can be a single value or a choice from a dropdown menu.
How to design an eCRF
Designing an electronic case report form is a critical step in running efficient and precise clinical trials. An eCRF is a digital template structured to capture and organize essential data from study participants. By spending time on planning the designing process of an eCRF, researchers can improve data collection and enhance data integrity.
Step 1: Craft a hypothesis
The main purpose of a clinical study is to gather data concerning variables relevant to your research hypothesis. Before designing an eCRF, define the study requirements, such as the type of data to be collected, the number of study visits, the study population, and the data analysis plan.
This step helps to identify the required data fields and the logical flow of data within the eCRF. Data fields must be determined based on the study requirements, including data types, validation rules, and edit checks. Data fields include text, numeric, date/time, or dropdown lists.
Step 2: Define the data collection strategy
To ensure that your clinical trial data is accurate and relevant, create a data collection plan that outlines the specific questions that need to be asked and how they’ll be formatted on an eCRF.
What kind of data can be included in eCRF?
An eCRF can include information such as:
- Patient characteristics and demographic data.
- Clinical study site and patient's treatment group.
- Patient health status, history, and vital sign measurements.
- Treatment effects/use.
- Patient lab reports and test results.
- Readings from patient-attached medical devices (e.g. blood pressure, heart rate, oxygen saturation, blood glucose level, etc.).
Step 3: Design the eCRF
Using an EDC system, you can design an eCRF. This involves creating data entry screens, validation rules, and edit checks. Once the data fields are identified, determine the necessary layout of the eCRF. This includes various data visualization processes like the arrangement of data fields, the use of tabbing or scrolling, and the use of color-coding or other visual aids.
Step 4: Test the eCRF
The eCRF needs to be tested to ensure that it works as expected. This includes testing the data entry screens, edit checks, and validation rules.
Step 5: Validate the eCRF
Validate the eCRF to guarantee that it meets regulatory requirements. This involves testing the system against predefined acceptance criteria and documenting the results.
Step 6: Implement the eCRF
Once the eCRF is validated, you can deploy it to study sites. This involves providing access to the eCRF, training study personnel on its use, and ensuring the data is collected, transformed, and transmitted securely.
Step 7: Observe the eCRF
Monitor the eCRF to ensure that it’s used correctly. This involves conducting regular data reviews, resolving queries, and updating the eCRF.
Tips for designing an effective eCRF:
- Keep the design straightforward and user-friendly.
- Emphasize the collection of quantifiable data whenever possible for more precise analysis and insights.
- Make certain data fields mandatory to capture all necessary information to reduce the chances of missing or incomplete data.
- Implement clear rules and validation checks for inputs to prevent errors and maintain data accuracy.
- Develop reusable eCRF templates or blocks to save time and effort when creating similar forms for different studies or research projects.
Benefits of eCRFs
Adopting electronic case report forms has revolutionized data collection in clinical trials and research studies. Unlike traditional paper-based methods, eCRFs offer numerous benefits that enhance data quality, streamline processes, and improve efficiency.
Consider these benefits if you are planning to implement an eCRF:
- Detailed data collection. eCRFs allow a thorough understanding of specific events, conditions, or outcomes, offering researchers and healthcare providers a comprehensive view of patterns over time for statistical analysis.
- Improved data accessibility. Standardization facilitates easier data access and data exchange among researchers, clinicians, and other authorized personnel, which promotes collaboration and enables evidence-based decision-making. Real-time data access supports transparency by allowing clinicians to monitor and report on the progress of research studies, outcomes, or quality improvement initiatives.
- Enhanced data quality. Standardized data entry methods, automated validation, and comprehensive data quality checks minimize errors or discrepancies and improve the accuracy and reliability of observations.
- Compliance with standards and regulations. Most eCRFs comply with data protection regulations and privacy standards, which safeguard sensitive information, promote ethical data handling practices, and build trust.
Challenges of eCRFs
While electronic case report forms offer significant advantages, they also come with specific challenges that researchers and study coordinators should know.
Here are some common challenges associated with eCRFs:
- Technical complexity. Working with eCRFs requires familiarity with the chosen electronic data capture system, which can be challenging, especially for those with limited technical skills. Integrating eCRFs with other clinical trial systems can also present challenges.
- Data privacy and security. Protecting participant data is crucial, and ensuring compliance with data protection regulations to prevent data breaches is difficult.
- Technical issues and downtime. Technical glitches, such as system downtime or connectivity issues, can disrupt the functioning of eCRFs.
- Cost considerations. While eCRFs can lead to long-term cost savings, the initial investments in software licenses, infrastructure, training, and ongoing maintenance can be substantial and pose budgetary constraints for smaller research organizations.
How is data collected in EDC software?
Data collection practices vary significantly between clinical studies because each has a different purpose. But EDC systems allow clinical trial sponsors to design customized eCRF forms to ensure that researchers collect the necessary data per the stated research hypothesis and data collection plan.
To better understand how electronic data capture fits into the clinical study workflow, let's look at three methods researchers use to input data into EDC software:
- Direct data entry allows researchers to log in to the EDC software, open the relevant eCRF for the study, and then add the clinical data to the system, which immediately becomes available for other stakeholders to review.
- Transcription from paper or electronic sources. In this case, researchers can complete paper CRF forms and then transcribe those into the EDC system. This is not recommended because errors made on a paper can be transferred to the electronic format, leading to data errors and defeating the purpose of using an eCRF over a paper CRF.
- Automatic transmission. Modern EDC systems can receive data transmissions from electronic patient-reported outcome (ePRO) instruments and connected medical devices and help in automating data entry portions.
The best electronic data capture software
EDC software is a computerized system that captures clinical trial data electronically. It creates, stores, and manages eCRFs. EDC software typically includes features such as data validation and verification, query management, and reporting tools.
So, while paper CRFs are a traditional way of capturing data, eCRFs created with EDC software are becoming popular for data capture in clinical trials.
For software to be included in this category, it needs to:
- Comply with regulatory requirements, such as the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines.
- Be intuitive so that users can easily enter data without making errors.
- Possess the ability to add or remove data fields, create new forms, and set up user roles and permissions.
- Include robust security features to protect the confidentiality and integrity of the data. This includes user authentication, data encryption, and audit trails.
- Integrate with other systems, such as electronic health record (EHR) software and clinical trial management systems (CTMS), to improve data collection and management.
- Offer users comprehensive support and training to ensure they can navigate the software effectively and troubleshoot any issues.
- Be cost-effective, taking into account the total cost of ownership over the entire course of the clinical trial.
* Below are the top 5 leading electronic data capture software solutions from G2’s Summer 2023 Grid® Report. Some reviews may be edited for clarity.
1. Medrio EDC
Medrio EDC, along with its complementary eClinical solutions, including ePRO, RTSM, eConsent, and Direct Data Capture (DDC), has gained the trust of leading pharmaceutical, biotech, CRO, animal health, medical device, and diagnostic companies since 2005. Their reputation is built not only on trust, but also on exceptional efficiency.
The platform has skip logic and edit checks as standard features, ensuring streamlined data collection. Additionally, their study build timeline is an impressive 75% faster than the industry average, saving valuable time in the research process.
What users like best:
"With a straightforward design, it is easy to navigate and simple to understand. Additionally, ensuring that queries and information are easily accessible allows users to retrieve the answers they seek quickly."
- Medrio EDC Review, Ammara C.
What users dislike:
"Studies demanding complex design cannot be programmed in Medrio. Edit check writing is an easy, but lengthy job, as each time, one needs to select each EDC object from the dropdown and drag it to the right place. It would be preferable if we do this using quick edit."
- Medrio EDC Review, Vaibhav K.
2. Medidata Rave
Medidata Rave is a cutting-edge cloud-based clinical data management system that revolutionizes capturing, managing, and reporting clinical research data. Designed to enhance efficiency and accuracy, Medidata Rave allows users to electronically record patient information, including visit details, laboratory results, and adverse events.
What users like best:
"It ensures the independent maintenance of all CRF versions, helps achieve the highest level of flexibility, offers extensive flexibility for database design, provides application programming interface (API) support, generates standard and custom reports, incorporates solutions like target source data verification (TSDV), safety gateway, batch uploader, and clinical views. Additionally, it is easy to deploy and offers exceptional user experience and comprehensive features for site and query management."
- Medidata Rave Review, Mohit J.
What users dislike:
"We have limited control over the visual appearance of form design. Additionally, we cannot utilize front-end queries such as JavaScript while programming."
- Medidata Rave Review, Suresh K.
3. Viedoc
Viedoc is an innovative solution designed to expedite clinical trials in the life science industry. Since its establishment in 2003, Viedoc has made a significant impact, enabling over 1 million patients from more than 75 countries to participate in studies powered by their technology. This demonstrates the widespread adoption and trust in Viedoc's solutions within the research community.
What users like best:
"The Viedoc team demonstrated exceptional knowledge, competence, and helpfulness throughout implementing our EDC for a new study. They provided invaluable advice and consistently delivered high-quality results within the agreed-upon timelines. Their prompt responsiveness and flexibility in addressing our questions and requests were commendable. Working with them was an awesome experience."
- Viedoc Review, Irina W.
What users dislike:
"Improvements needed include immediate testing of edit checks, subject-level updates, restricted editing of system-built pages, and programming capabilities on system-generated pages."
- Viedoc Review, Nazeli S.
4. Drug Safety Triager
Drug Safety Triager is an advanced, validated pharmacovigilance (PV) literature review software. It enhances patient safety by streamlining the monitoring process, leveraging intelligent automation through a machine learning engine - DELVE. Simplifying the review process, it enables teams to prioritize patient well-being effectively.
What users like best:
"As a nurse or healthcare professional, the Drug Safety Triager proves to be beneficial. However, its most outstanding feature lies in its regular updates. Given the continuous production of new medications and the importance of monitoring their efficacy and safety, Triager's ability to screen and assess drugs collectively and individually becomes not just helpful but an absolute necessity in our field. Even if it wasn't integrated into our EMR system, I would love to have it as a standalone tool for work. I cannot emphasize enough how essential the Drug Safety Triager is; I have nothing but positive things to say about it."
- Drug Safety Triager Review, Sheena C.
What users dislike:
"To maximize the effectiveness of the Drug Safety Triager, the training material must be user-friendly and easily understandable. Furthermore, enhancing the data analysis capabilities to provide a broader range of signals for analysis from the available resources would significantly amplify its impact. By incorporating these improvements, the Drug Safety Triager can offer users a more intuitive and insightful experience, enabling them to make informed decisions with greater confidence."
- Drug Safety Triager Review, Sanjib M.
5. OpenClinica
OpenClinica is a comprehensive solution that empowers data managers, clinical researchers, and study participants through efficient clinical data management and automation.
What users like best:
"I utilize OpenClinica 4 as a Data Manager, which has proven to be an exceptional choice. OpenClinica 4 is a cutting-edge and highly intuitive system encompassing all the necessary functionalities for my role. The standout feature for me is the Insight tool, which offers invaluable insights and analysis capabilities. Additionally, the support team consistently provides prompt and knowledgeable assistance, ensuring a seamless experience."
- OpenClinica Review, Giorgos T.
What users dislike:
"The end user interface lacks user-friendliness, but incorporating newer technologies and a cascading style sheet (CSS) framework can greatly improve its usability and make it mobile-friendly as well."
- OpenClinica Review, Shilkumar N.
Pen and paper just won't cut it
Electronic case report forms offer a versatile solution for collecting and managing data in clinical trials. eCRFs address many data collection challenges that often arise during clinical studies, such as managing data across multiple locations.
While CRFs are still in use, clinical studies have been seen to transition to eCRFs rapidly. With eCRFs, all those involved in the clinical study process can easily access and share information, which leads to more efficient and effective research.
Data collection, storage, and management are essential in many industries – especially healthcare. Learn more about how electronic data capture helps manage clinical trial data.

Devyani Mehta
Devyani Mehta is a content marketing specialist at G2. She has worked with several SaaS startups in India, which has helped her gain diverse industry experience. At G2, she shares her insights on complex cybersecurity concepts like web application firewalls, RASP, and SSPM. Outside work, she enjoys traveling, cafe hopping, and volunteering in the education sector. Connect with her on LinkedIn.